Many solutions contain one component, called the solvent, in which other components, called solutes, are dissolved. An aqueous solution is one for which the solvent is water. The concentration of a solution is a measure of the relative amount of solute in a given amount of solution. Concentrations may be measured using various units, with one very useful unit being molarity, defined as the number of moles of solute per liter of solution.
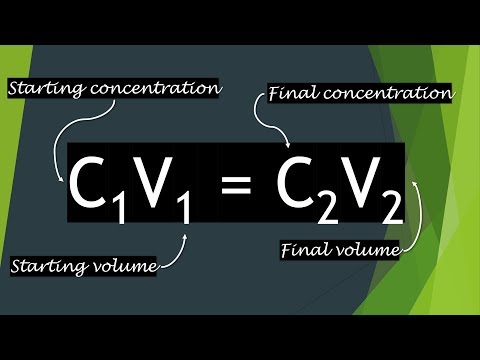
The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. An aqueous solution consists of at least two components, the solvent and the solute .
Usually one wants to keep track of the amount of the solute dissolved in the solution. One could do by keeping track of the concentration by determining the mass of each component, but it is usually easier to measure liquids by volume instead of mass. To do this measure called molarity is commonly used. Molarity is defined as the number of moles of solute divided by the volume of the solution in liters. To make a solution from solid solutes, first calculate how many moles of solute are in the desired solutions .
Calculate the amount of solid you need in grams using the moles needed and the molar mass of the solute and weight out the needed amount. Transfer the solute to a container and add a small amount of solvent. Once the solute has dissolved, add the remaining solvent to make the solution of the desired volume and mix thoroughly. It is important to note that the molarity is defined as moles of solute per liter of solution, not moles of solute per liter of solvent. To get around this problem chemists commonly make up their solutions in volumetric flasks. These are flasks that have a long neck with an etched line indicating the volume.
The solute is added to the flask first and then water is added until the solution reaches the mark. The flasks have very good calibration so volumes are commonly known to at least four significant figures. 2-furoic acid has an increased solubility in boiling water compared to cold water, as shown by the solubility data in Figure 3.20. The quantity of hot solvent needed to dissolve this sample can be calculated using the compound's solubility in hot water, as shown below.
This represents the "minimum amount of hot solvent" needed for the crystallization. Different chemical compounds dissolve in solutes in varying degrees. Some compounds, such as the strong acid hydrochloric acid , dissociate completely in solution into ions. Others, like the weak base ammonia , only partly dissociate. Yet other compounds like alcohol do not dissociate at all and remain compounds. Laboratory reactions often involve acids and bases, which are covered in more detail here.
Convert the resulting moles of solute back to molarity by dividing by the total volume, in liters, of solution used in the reaction. If the concentration is not given but the molar mass and volume are, use density (grams/Liter) to find the amount of solute in grams, then convert it to moles. Scientists sometimes use molality to measure concentration because liquid volumes change slightly based on the temperature and pressure.
Mass, however, stays the same and can be measured accurately using a balance. Commercial concentrated products are usually expressed in mass percent; such as commercial concentrated sulfuric acid, which is 93-98% H2SO4 by mass in water . Water is the most common solvent, used for dissolving many compounds or brewing coffee. Other common solvents include turpentine , acetone , and ethanol .
Such solvents usually contain carbon and are called organic solvents. Solutions with water as the solvent are called aqueous solutions; they have special properties that are covered here. The volume units must be the same for both volumes in this equation. In general, M1 usually refers to as the initial molarity of the solution.
V1 refers to the volume that is being transferred. M2 refers to the final concentration of the solution and V2 is the final total volume of the solution. The equation for calculating Molarity from the moles and volume is very simple. Just divide moles of solute by volume of solution. How many grams of hydrochloric acid are required to react completely with 4.30 grams of zinc?
I got 4.80g HCl for the answer but do not know how to do part 2 which is, How many molecules of a gas will be produced? Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. Every solid has partial solubility in the solvents used, even at cold temperatures. In Figure 3.19 the cold solvent surrounding a yellow solid is tinted yellow as some compound dissolves.
Solids that appear insoluble in a solvent do in fact have a portion of material that dissolves. This is analogous to how "water-insoluble" ionic compounds (such as \(\ce\) have a non-zero solubility product constant \(\left( K_\text \right)\). Multiply the moles of NaCl by its molar mass (58.44 g/mol) to find the grams of solute needed. If the amount in mol of a solute in a given volume of solution is known, its mass can also be calculated. Organic chemical reactions refer to the transformation of substances in the presence of carbon.
This lesson will explore organic chemical reactions dealing with hydrocarbons, including addition, substitution, polymerization, and cracking. We need to know the number of moles of sulfuric acid dissolved in the solution and the volume of the solution. Use the simulation to explore the relations between solute amount, solution volume, and concentration and to confirm the dilution equation. The relative amount of a given solution component is known as its concentration. Often, though not always, a solution contains one component with a concentration that is significantly greater than that of all other components.
This component is called the solvent and may be viewed as the medium in which the other components are dispersed, or dissolved. Solutions in which water is the solvent are, of course, very common on our planet. A solution in which water is the solvent is called an aqueous solution. Making a solution of a certain concentration from a stock solution is called a dilution. When diluting a solution, keep in mind that adding a solvent to a solution changes the concentration of the solution, but not the amount of solute already present.
Multiply the concentration (0.5 mols/Liters) by the volume of solution you want (0.5 Liters) to find the moles of NaCl you need. A solution consists of two or more substances dissolved in a liquid form. Think of it as comparing a cup of sugar water and a cup of water with lego blocks in it. The solute is the substance dissolved in the solution, and the solvent is the substance doing the dissolving.
Remeber that the number of moles of solute does not change when more solvent is added to the solution. Concentration, however, does change with the added amount of solvent. 72.0 mL of a 1.60 M solution is diluted to a total volume of 278 mL. A 139-mL portion of that solution is diluted by adding 155 mL of water. Where M1 and V1 are the molarity and volume of the original stock solution and M2 and V2 are the molarity and volume of...
The standard enthalpy of formation or the standard heat of formation is used in calculations to determine changes in enthalpy. Study the energy of chemical reactions and the explanation and calculations for standard enthalpy of formation. Learn about nuclear fusion, nuclear fission, tracers, imaging, and practical applications of nuclear chemistry. Learn how to find the molarity of a solution or the moles of gas in a given volume using stoichiometry.
Colligative properties are important to determine molar mass as related to vapor pressure, boiling point, freezing point, and osmotic pressure. Examine freezing point depression to understand the process for finding molar mass. Calculate the minimum volume of 1.10 \ M \ CuSO_4 solution that is required to ensure complete reaction of 1.04 \ gram of Fe . Since you do not know which reaction will be the one to occur, you must base the calculation on whichever of the equations requires more CuSO_4 solution. If we had not retained this guard digit, the final calculation for the mass of NaCl would have been 77.1 g, a difference of 0.3 g.
Calculate the molarity of 6.52 g of CoCl2 (128.9 g/mol) dissolved in an aqueous solution with a total volume of 75.0 mL. A solute is a component of a solution that is typically present at a much lower concentration than the solvent. Solute concentrations are often described with qualitative terms such as dilute and concentrated .
We have previously defined solutions as homogeneous mixtures, meaning that the composition of the mixture is uniform throughout its entire volume. Solutions occur frequently in nature and have also been implemented in many forms of manmade technology. Concentration is the measure of the amount of solute in a certain amount of solvent.
Knowing the concentration of a solution is important determining the strength of an acid or base , among other things. When there is so much solute present in a concentration that it no longer dissolves, the solution is saturated. Now notice in the balanced equation that 2 moles of HCl reacts with one mole of Mg, therefore we need two times as many moles of HCl as we have of Mg. The relative formula mass of a compound is calculated by adding together the relative atomic mass values for all the atoms in its formula. Moles are units used to measure substance amount.
Hybridization is the process of mixing two or more atomic orbitals to create new covalently bonded orbitals in molecules. However, hybrid orbitals and pure atomic orbitals have different molecular shapes. Learn about orbital hybridization theory, valence bond theory, the difference between sigma and pi bonds, and how to predict the molecular shape of atomic orbitals. Rate law is defined as the relationship between the rate of reaction and the concentration of the reactants.
Learn about rate law, rate constant, and how to use rate law equations to determine reaction order for one or two reactants. Learn the concepts of molar volume and standard molar volume. See how to calculate molar volume and use the correct molar volume units. In acid-base chemistry, titrations can be used to determine the concentration of an unknown solution. Explore titration, titrant, neutralization reaction and equivalence point, and how to perform a titration and interpret a titration curve. M1 and V1 are the concentration and volume of the original solution to dilute; M2 and V2 are the desired concentration and volume of the final solution.
Solutions used in the laboratory are usually made from either solid solutes or stock solutions. There are two solutions involved in this problem. Notice that you are given two concentrations, but only one volume.
Solution #1 is the one for which you have only concentration - the solution that is already sitting on the shelf. Solution #2 is the one for which you have both concentration and volume - the solution that you are going to prepare. The substances on the left hand side of the equation, i.e. the reactants, the substances that you are going to add together to make a chemical reaction. From balanced equation, each mole KOH reacts with 1 mole HNO3. To prepare this solution you would use a pipet to transfer 30 ml of stock solution to a 250 ml volumetric flask.
Add distilled water almost to the line on the neck of the flask, stopper, invert and swirl. Use a dropper to add distilled water to the line on the neck of the flask and mix again by inverting and swirling. Every solution has a solubility product constant to represent its state in dynamic equilibrium. Learn about the solubility equilibrium and how to use a Ksp in calculations, determining Ksp from ion concentration and vice-versa.
An aqueous solution of an acid is so weak that... The quantity of solvent used in crystallization is usually kept to a minimum, to support the goal of recovering the maximum amount of crystals. In the case of reactions involving ions , eliminate spectator ions from the net ionic equation. Scientists often use molarity to measure concentration. When the solvent volume is doubled, the quantity of material dissolved in the mother liquor also doubles, as shown in the calculation below.
A less common unit for concentration is called molality. Now cross multiply through the equation and solve for X moles of HCl I got 24.1 moles of HCl needed. Our certified Educators are real professors, teachers, and scholars who use their academic expertise to tackle your toughest questions. Educators go through a rigorous application process, and every answer they submit is reviewed by our in-house editorial team. Graham's Law helps explain how gas particles move through the air. Learn about Graham's Law, including the processes of diffusion and effusion, and explore how to use the law to solve problems.
Isotopes are variations of the same element with differing numbers of neutrons and, subsequently, different atomic masses. Learn how scientists consider isotopes when they calculate average atomic mass. When a substance absorbs or gives out heat, the measurement of the heat transfer is calorimetry. Explore the meaning of calorimetry, the units of heat, specific heat capacity, and calorimetry and heat calculations.
What is an ideal gas and the law relating to the pressure, volume, and temperature of the ideal gas? Learn about an ideal gas constant and its characteristics. Learn about colligative properties and their equations.






















No comments:
Post a Comment
Note: Only a member of this blog may post a comment.